锌负极/水电解液界面上存在着一系列常见的寄生副反应,极大的阻碍了水系锌基器件的大规模储能开发。在锌负极上进行人工聚合物涂层人是解决这一问题的有效途径。然而,大多数亲水聚合物层在长期循环过程中存在被水溶液溶解的风险。传统的与金属具有较强结合力的涂层,往往具有疏水性,极大增加了锌电极/水电解液的界面电阻,进一步抑制了界面离子的迁移。
近日,西南石油大学何显儒团队与松山湖材料实验室王欣团队合作,通过平衡粘结性与亲水性,设计了一种丙烯酸酯型的亲水粘接性聚合物涂层(HAC),作用于锌负极表面。令人鼓舞的是,该涂层不仅可以增强锌离子迁移动力学,同时可以调节其沉积/溶解行为,最终实现了对枝晶和副反应出色的抑制效果。研究表明,HAC改性后的锌对称电池具有长循环稳定性,即使在较大电流密度5 mA cm-2,容量5 mA cm-2 h下仍表现出超过800小时的长循环寿命。此外, 组成的HAC锌-活性炭 (AC) 混合超级电容器也表现出了优异的电化学性能,包括将工作电压提高至2.0 V以及出色的循环稳定性,在10 A g-1下,循环14000次后仍可保持100%的初始比电容。

Fig. 1. Physicochemical properties of HAC. (a) and (b) Contact angles between Bare Zn/HAC-modified Zn electrode and aqueous electrolyte at 5 min; (c) FTIR spectra of HAC before and after plating of Zn2+; (d) Nyquist plots of Zn-Zn symmetric cells with Bare Zn/HAC-modified Zn electrode; (e) Transference number of Zn2+ before and after coating HAC; (f) Stress-strain curve of HAC film.

Fig. 2. Electrochemical stabilities between Zn anode (Bare Zn and HAC-modified Zn) and aqueous electrolyte investigated via galvanostatic electro-plating and stripping processes. (a), (b) and (c) Zn-Zn symmetric cells with Bare Zn and HAC-modified Zn cycled at a current density of 5 mA cm-2, 10 mA cm-2, and varying current densities. Insets of (a) and (b) are the magnified view of voltage profiles. (d) and (e) Top-view SEM images of HAC-modified Zn and Bare Zn surface after cycling at a current density of 1 mA cm-2 with a capacity of 1 mA h cm-2 for 100 h. (f) XRD patterns of Bare Zn and HAC-modified Zn anodes before and after cycling at a current density of 1 mA cm-2 with a capacity of 1 mA h cm-2 for 100 h.

Fig. 3. Electrochemical reversibility between Bare Zn and HAC-modified Zn anodes with aqueous electrolyte. (a) Tafel curves of Bare Zn and HAC-modified Zn. (b) Chronoamperograms (CAs) of Bare Zn and HAC-modified Zn under a -200 mV overpotential. (c) Initial nucleation overpotentials of Zn plating on Ti foil in Bare Zn and HAC-modified Zn asymmetric cells at 5 mA cm-2. (d) Coulombic efficiency (CE) of Zn plating/stripping in Bare Zn-Ti and HAC-modified Zn-Ti cells at a current density of 5 mA cm-2 for 10 min and (e,f) corresponding voltage profiles at various cycles.
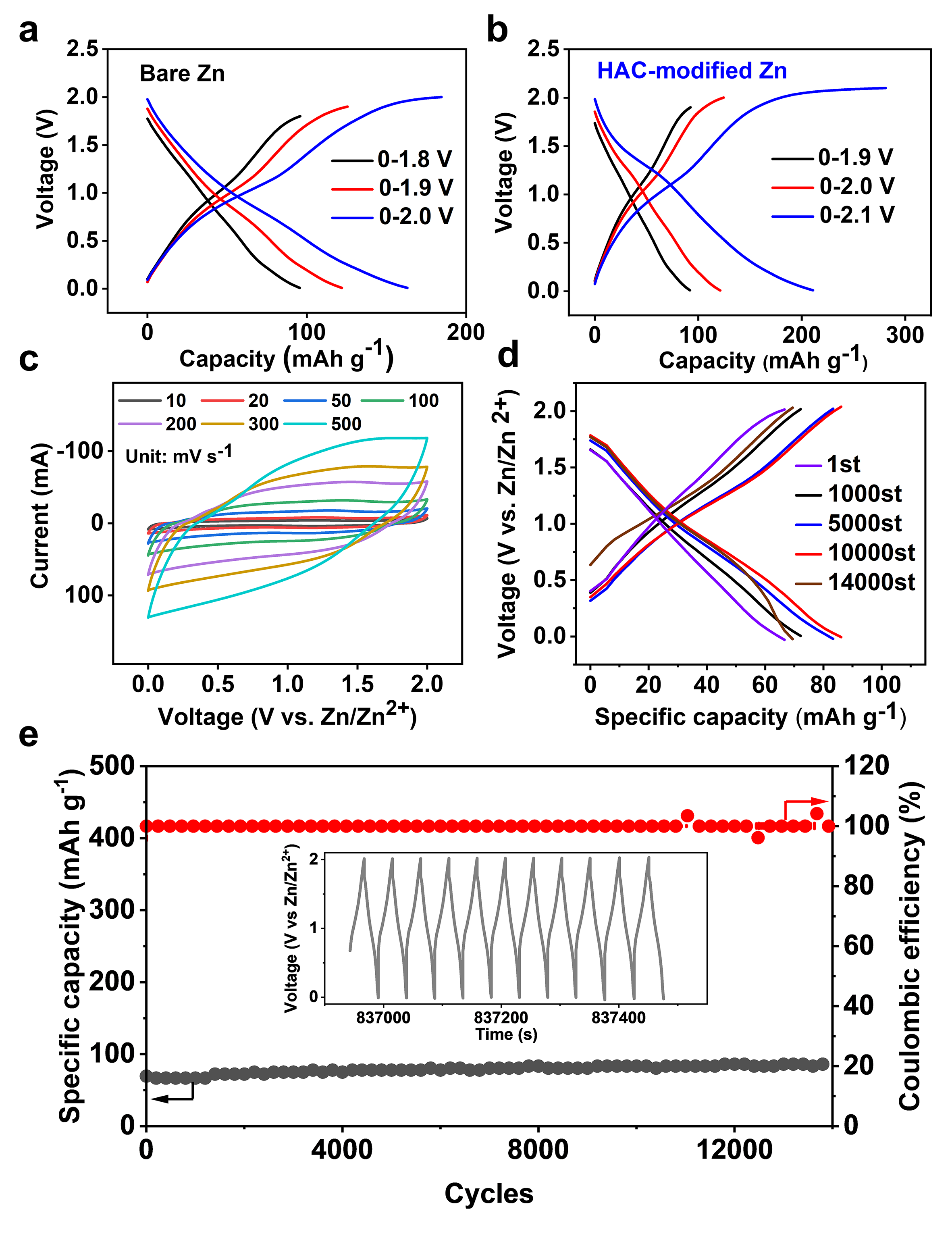
Fig. 4. Electrochemical performance of Zn-AC ion hybrid supercapacitors. (a) and (b) Galvanostatic capacity-voltage curves of the Zn-AC hybrid capacitors with Bare Zn and HAC-modified Zn anodes showing a 0.1 V increase in electrochemical window with the introduction of HAC. (c) CV curves at scan rates indicated. (d) and (e) Galvanostatic capacity-voltage curves and long-term cycling stability at 10 A g-1.

Fig. 5. (a) Schematic illustration of morphology evolution for both the symmetric cells with different electrodes during stripping/plating. (b) Comparison of performance among coating-modified Zn anode devices (see Table S1 for references). The selected references have a current density greater than 3 mA cm-2. (c) Comparison of electrochemical performance of cells with Bare Zn and HAC-modified Zn anodes.
相关工作以 “Robust Zn anode enabled by a hydrophilic adhesive coating for long-life zinc-ion hybrid supercapacitors”为题发表于《Chemical Engineering Journal》上。论文第一作者为西南石油大学博士研究生牛奔和厦门大学博士研究生李振刚,西南石油大学何显儒教授和松山湖材料实验室王欣研究员为论文共同通讯作者。
原文链接:https://doi.org/10.1016/j.cej.2022.136217
- 山东农大刘峰/张大侠团队 AFM:界面聚合过程中油相亲水性对膜形成动力学的多角度影响 2026-02-11
- 杭师大朱雨田教授、陈建闻副教授/南开刘遵峰教授 AFM:基于多尺度亲水-疏水界面超快响应与超大形变的湿气响应Janus纤维驱动器 2025-10-18
- 广西大学林宝凤教授 IJBM:XSBR介导的生物质聚合物 - 一种解决天然多糖基薄膜低韧性和高亲水性的新策略及在食品包装中的应用 2025-06-23
- 川大梁坤能副教授和邓怡研究员团队 Nano Lett.:特洛伊木马生物异质结赋予粘接性水凝胶强大的抗菌和传感性能用于感染皮肤再生 2024-12-11
- 江苏科大赵正柏、李为立课题组《Chem. Mater.》:一种具有高导热、高柔韧和高粘接性的石墨烯/聚烯烃弹性体薄膜用于芯片散热 2023-03-23
- 广州大学林璟、陕理工郑楠《Adv. Compos. Hybrid Mater.》:一种可实现快速自修复、防污的PFPMS复合材料 2022-11-21
- 浙江大学计剑、张鹏团队 JACS:选择性细胞阻抗调控细胞-材料界面相互作用 2025-03-07
