糖尿病正成为越来越严重的公共健康问题,全球糖尿病患者总数已超过5亿。I型和重症II型糖尿病的治疗离不开胰岛素,精准控制胰岛素的给药剂量对患者的治疗至关重要。剂量过低起不到理想的治疗效果,过高将导致低血糖,可能危及患者生命,其中以夜间低血糖最为致命,约占糖尿病总致死人数的6%。目前主要采用分次注射胰岛素的方法改善夜间低血糖问题,但是该方法需要人工调节注射的胰岛素剂量,操作较为麻烦。因此,设计具有葡萄糖响应性能的胰岛素递送体系,根据血糖浓度实时自适应地控制胰岛素的释放对夜间血糖控制具有重要意义。
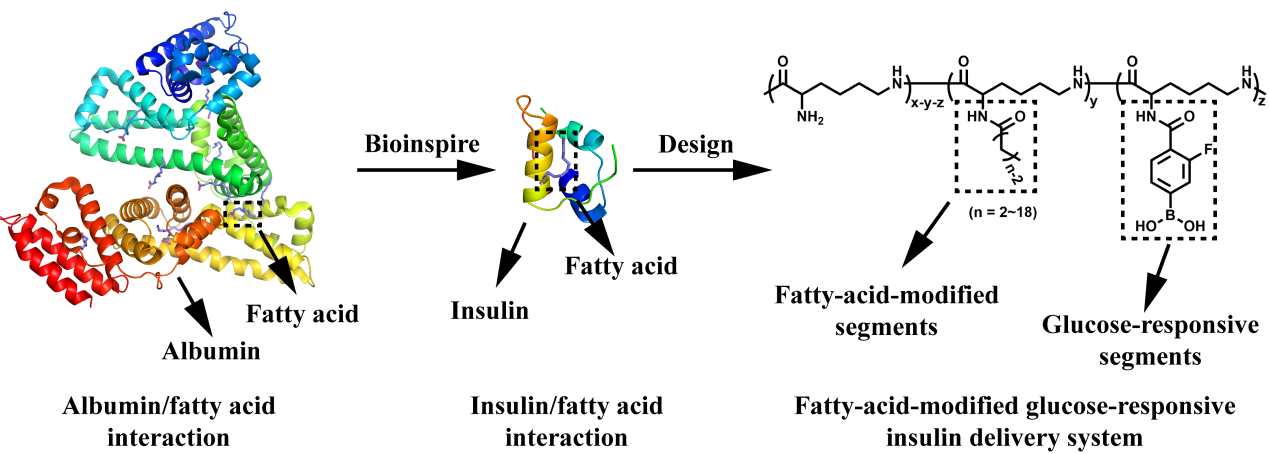
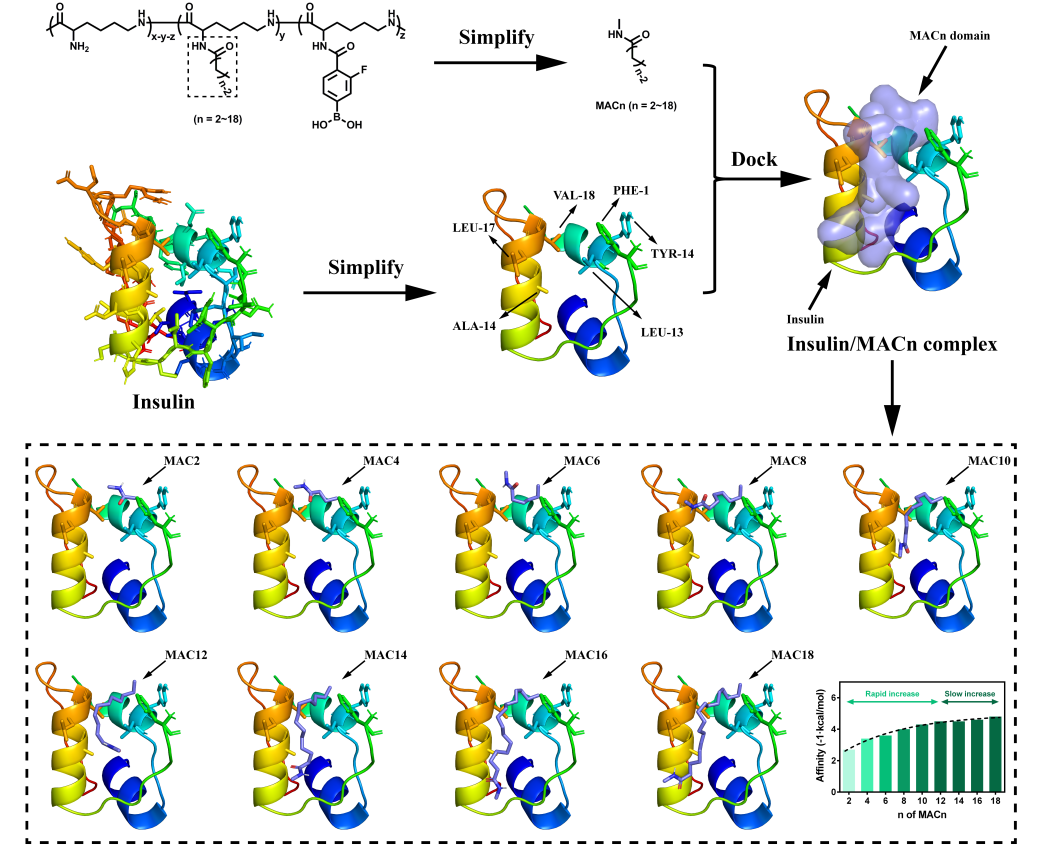
近日,浙江大学王立教授和俞豪杰副教授团队受脂肪酸/白蛋白疏水相互作用的启发,基于分子对接技术设计了一种脂肪酸与苯硼酸衍生物双修饰的纳米粒子用于胰岛素负载与血糖浓度响应释放,其设计思路如Figure 1所示。通过分子对接技术将脂肪酸基团片段与胰岛素的相互作用域可视化,并结合数据分析发现随脂肪酸基团链长增长,其与胰岛素间的疏水作用变强(Figure 2),这有利于抑制胰岛素的突释行为,从而避免胰岛素释放过快导致的低血糖症状。
通过实验验证了正丁酸、正己酸、正辛酸、正癸酸和月桂酸对纳米粒子自组装行为的影响,发现脂肪酸基团链长适中时,纳米粒子自组装形貌更规整,有利于胰岛素的负载(Figure 3)。制备了9种脂肪酸与苯硼酸衍生物双修饰纳米粒子并筛选出性能最优的纳米粒子C10MS,其胰岛素负载量为0.17 g胰岛素/g载体。C10MS能抑制胰岛素的突释行为,表现出稳定的糖敏胰岛素释放性能。

Figure 3. SEM images of (a) C4MS, (b) C6MS, (c) C8MS, (d) C10MS and (e) C12MS and the schematic diagrams (bar = 500 nm).

Figure 4. The glucose-responsive insulin-releasing mechanism.

Figure 5. (a, b) The hypoglycemia-avoiding performances of the anti-diabetes agents evaluated on healthy rats with (c) the statistical analysis. (d, e) The 8-h hyperglycemia-ameliorating and hypoglycemia-avoiding performances of the anti-diabetes agents evaluated on diabetic rats with (f) the statistical analysis. (g) The 14-h hyperglycemia-ameliorating and hypoglycemia-avoiding performances. The data of “Diabetic control” were shown as the means ± SD (n = 3). The data of “Healthy control”, “C6MS”, “C8MS”, “C10MS”, “INS”, “Det-INS”, “INS(H) + Det-INS(L)” and “INS(L) + Det-INS(H)” were shown as the means ± SD (n = 5). The statistical analyses were performed by two-tailed Student''s t-test. * P < 0.05 and ** P < 0.01.

Figure 6. (a) The MTT assays and live-dead cell staining assays for CnMSs (n = 6, 8 and 10, scale bar = 200 μm). (b) The hemolysis tests for CnMS (n = 4, 6, 8, 10 and 12) and the routine blood test for C10MS. (c) The in vivo fluorescence test on diabetic rats by using Cy5-labeled C10MS. (d) Histological analyses of C10MS on hearts, livers, spleens, lungs, and kidneys (scale bar = 100 μm). The data of MTT assays were shown as the means ± SD (n = 6). The data of hemolysis tests were shown as the means ± SD (n = 3). The data of the routine blood test were shown as the means ± SD (n = 4).
论文链接:https://doi.org/10.1016/j.jconrel.2022.10.044
- 浙江大学伍广朋教授课题组 Macromolecules:氢键驱动β-内酯开环聚合制备聚羟基脂肪酸 2025-03-03
- 清华大学陈国强教授、吴琼副教授 Adv. Sci.: 以聚羟基脂肪酸酯为例探究细胞体积大小对工业生产的影响 2025-02-22
- 天科大马晓军/李冬娜等 Biotechnol. Adv. 综述:木质纤维素生物质生产聚羟基脂肪酸酯的进展及展望 2025-01-02
- 吉林大学钱虎军教授团队 PRL:单链纳米粒子突破高分子材料“强度-韧性-加工性”三难困境 2025-10-15
- 浙江大学王立教授、俞豪杰教授团队 JCIS:用于安全磁共振成像和刺激响应药物递送的纳米粒子 2025-09-09
- 华工殷盼超/广工尹家福团队 Nano Lett.:软纳米粒子多时空动力学解析 2025-08-21
- 浙江大学王立教授、俞豪杰教授团队 Carbohyd. Polym.:基于糖敏水凝胶的智能长效胰岛素递送微针贴 2024-11-21
