Cun-Jiang Song1*, Shu-Fang Wang2, Mitsuyo Sano3, Shigeya Takeuchi3
1 Key Laboratory of Bioactive Materials for Ministry of Education, Department of
Microbiology, Life Science College, Nankai University, Tianjin 300071, P. R. China.
Tel & Fax: +86-22-23503866; E-mail: songcj@nankai.edu.cn
2 State Key Laboratory of Functional Polymer Materials for Adsorption and Separation,
Institute of Polymer Chemistry, Nankai University, Tianjin 300071, P. R. China.
3 Faculty of Education, Toyama University, Gofuku, Toyama 930-8555, Japan.
Abstract: To assess the capacity of the natural environment for degrading biodegradable materials,the film-MPN method proposed previously was modified to estimate the numbers of PHB/V degrading microorganisms (degraders) in various environments. The Relationship between incubation and positive tubes was studied to determine the appropriate incubation period for the method. Numbers of aerobic PHB/V degraders were estimated in garden soil, paddy field soil, farm soil, river bank soil, infertile garden soil, river water, activated sludge, and seawater by the film-MPN method. Results were compared with those estimated by the clear-zone technique and showed that the film-MPN method was suitable for estimating the numbers of PHB/V degraders in the environments tested. On the other hand, biodegradability of injection molded PHB/V samples was investigated in several kinds of environments. The changes of weight were studied and results showed that biodegradability of PHB/V related to the numbers of PHB/V degraders in similar ecosystem in different regions. In different environments the biodegradability of PHB/V not only related to the number of PHB/V degraders, but also depended on whether there were conditions for the PHB/V degraders to grow and proliferate easily in the environment.
Key Words: Biodegradation; film-MPN method; PHB/V degrader; natural environment; injection molded PHB/V sample.
Introduction
The biodegradability of various environmental biodegradable materials has been reported by many groups [1-10]. The clear-zone technique is widely used to estimate numbers of biodegradable materials-degrading microorganisms (degraders) in various environments. In this method (For example PHA), PHA is dispersed on an agar plate as a milky suspension of powder. When PHA degraders are inoculated, they secrete PHA
depolymerases and extracellularly hydrolyze PHA to water-soluble products. Thus, a transparent clear-zone around a depolymerase-producing colony is created on the agar plate. By the technique, Jendrossek cultivated polymerdecomposing bacteria to isolate depolymerase [11]; Briese estimated total numbers of aerobic PHB degraders in activated sludge [12]; Mergaert isolated PHA degraders from soil and compost [13].Based on the principle of the technique, Matavulj and Molitoris found that 55% of terrestrial fungal isolates hydrolyzed PHB and / or PHB/V in cultured tubes [14].
Unfortunately, only a few biodegradable materials can be prepared as milky suspensions of powders. Most of other biodegradable materials, including poly (4-hydroxybutyrate) and all PHAMCL (monomer-containing 6-15 carbon atoms), form large rubber-like aggregations. Hence, the clear-zone technique cannot be used [8]. To solve the problem, many methods have been proposed. Nishida estimated the numbers of PHB and poly (3-propiolactone) degraders in different kinds of environments using a modified clear-zone technique by emulsifying polymers with a surfactant (Plysurf A 210G) [15, 16]. Horowitz and Sanders prepared a PHA emulsion with chloroform and an emulsifier (sodium dodecyl sulfate, SDS) [17]. Ramsay and Marchessault studied artificial granule suspension of PHA [18,19]. In all above-mentioned methods, PHA is emulsified with different types of emulsifiers.
However, Jendrossek suggested that emulsifier or surfactant inhibited bacterial growth and PHA depolymerase activity [8]. Therefore, some groups used a PHA-film as carbon source to isolate PHA degraders. Schirmer used a PHA film between the bottom and top agar layers and successfully isolated 28 PHA degraders [20]. We previously proposed a film-MPN method and estimated the numbers of PHB/V
degraders in two soil [21], and studied the biodegradability of several biodegradable materials by the method [22-25].
In the present study, we improved the film-MPN method, i.e. the film-MPN method applied to aquatic ecosystem was constructed. Numbers of PHB/V degraders were estimated in various kinds of environments. Furthermore, the relationship between biodegradability of injection molded PHB/V test pieces and the numbers of PHB/V degraders in several kinds of environments was investigated.
Materials and methods
Garden soil sample
Soil samples were collected from topsoil (0 – 10 cm in depth) in the campus of Toyama University, Toyama, Japan, which had no history of PHB/V exposure. Some of the soil properties were as follows: pH (H2O), 7.35; pH (KCl), 6.85; H2O, 21.5%; %C,3.01; %H, 0.66; and %N, 0.21.
Paddy field soil sample
Paddy field soil samples were collected from topsoil (0 –10 cm in depth) in a farm near Toyama University, Toyama Japan that had no history of PHB/V exposure. Someof the farm soil properties were as follows: pH (H2O), 5.48; pH (KCl), 4.09; H2O,17.03%; %C, 2.43; %H, 0.71; and %N, 0.25.
Farm soil sample
Farm soil samples were collected from topsoil (0 –10 cm in depth) in a farm in Toyama University, The brown lowland soil H2O%: 20.79%, C %: 2.02%, H%: 0.56%,N%: 0.31%, PH(H2O): 7.35.
Infertile garden soil sample
Infertile garden soil samples were collected from topsoil (0 –10 cm in depth) in the garden of Toyama Industrial Technological Centerfarm, Takaouka, Toyama, Japan. The Sandy soil, H2O%: 12.59%, C %: 0.42%, H%: 0.40%, N%: 0.04%, pH(H2O): 6.29.
River bank soil sample
River bank soil samples were collected from topsoil (0 -10 cm in depth) from the river bank of Zinzukawa River, near Toyama University, Toyama, Japan, which had no history of PHB/V exposure. Some of the river bank soil properties were as follows: pH (H2O), 5.91; pH (KCl) 4.59; H2O, 25.03%; %C, 2.04; %H, 0.61; and %N, 0.23.
Aerobic activated sludge
Aerobic activated sludge sample was obtained from the Hamakurosaki Water Purification Center, Toyama, Japan.
River water and seawater
River water was sampled from Zinzukawa River near Toyama University, Toyama,Japan and seawater was sampled from the Yokata Seacoast in Toyama Bay, Japan.
Preparation of PHB/V films
PHB/V powder and granules were obtained from Zeneca Co. Ltd., Japan, and 3HV mol% was 5%. To prepare the film, 0.3 g PHB/V was dissolved in 100 ml chloroform,and the solution was transferred to a polytetrafluoroethylene tray (14.5 cm in diameter×2.5 cm in height) covered with a Petri dish to slow the rate of evaporation in order toget even film. The solvent was allowed to evaporate overnight at room temperature [27].
The film was further dried under vacuum at 70 0C for 2 hours. The thickness of the filmwas 0.05 - 0.08 mm.
The film-MPN method to estimate numbers of PHB/V degraders
A medium was prepared with 0.033 M KH2PO4-Na2HPO4 buffer ( pH 6.8, 1 liter )containing 1 g NH4Cl, 0.5 g MgSO47H2O, 0.05 g ferric ammonium citrate and 0.005 gCaCl2·2H2O [4]. Then 0.05 g yeast extract and 0.1 g casein hydrolysate (vitamin-free and salt-free) were added [5]. 5 ml of the medium was poured into a tube (1.0 cm in diameter ×10 cm in height ) containing a piece of PHB/V film (1.0×7.0 cm, 0.015 -0.020 g), and the films were stood in the tube using a glass bar (0.5 mm in diameter×7.5 cm inlength ), which was sterilized by autoclaving at 110 0C for 30 min. The environmental samples were diluted at 101 ~1010 with the liquid medium (The seawater samples were diluted at 24~213.). 1 ml of each diluted solution was inoculated into eachof 5 tubes. The tubes were incubated at 28 0C under aerobic conditions in the dark. Thenumbers of positive-growth tubes were counted after the fixed time. A MPN statistical table was used to determine the growth code and to calculate the MPNs [28].
The clear-zone technique to estimate numbers of PHB/V degraders
Solid medium containing PHB/V powder as the carbon source was prepared by pouring an overlay of 5 ml of hot mineral agar solution containing 0.25% (w/v) polymer powder onto the preheated (37 0C) bottom layer of mineral agar (25 ml), resulting in the formation of an opaque top layer. Other components of the medium were the same as those mentioned above. The plates were incubated at 28 0C for 4 to 5 weeks [12], and the numbers of colonies with clear zones were counted after 5 weeks. ( see Fig. 1)
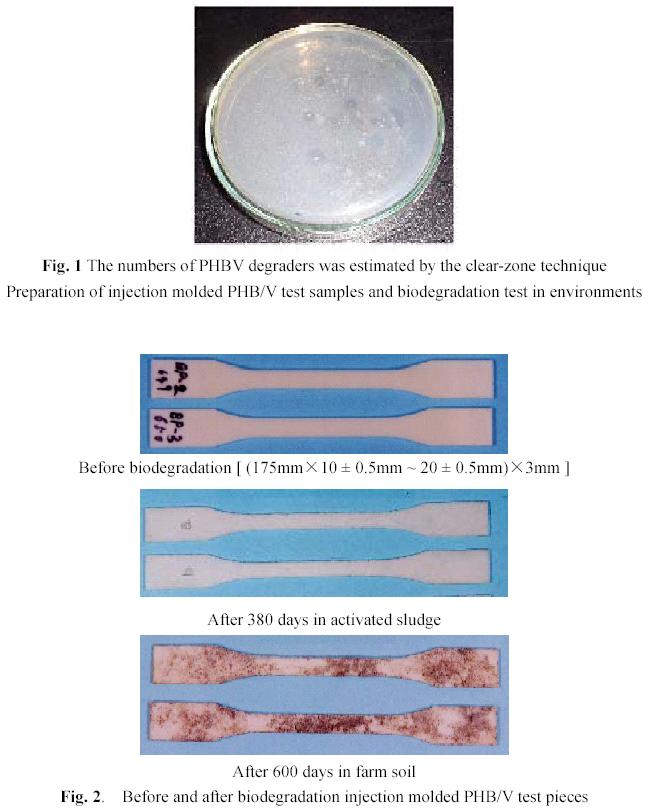
The JIS No.1 test pieces for dog-bone shape are prepared by injection molding machine FN1000-12A (NISEI JYUSHI KOUGYOU in Japan). The thickness of testpieces is 3 mm.
After the test pieces were treated under constant temperature and humidity ( 20 0C and 65% ) for a week, the test pieces were buried in the farm soil and infertile garden soil between 10 –20 cm depth and submerged in river water, seawater and activated sludge between 50 –100 cm depth. After 30, 60, 120, 240, 360, 480 and 600 days, test pieces were removed and cleaned, treated under constant temperature and humidity ( 200C and 65% ) for a week, weight losses were determined (see Fig. 2).
Results and discussion
Construction of film-MPN method applied to aquatic ecosystem
The key to construction of film-MPN method applied to aquatic ecosystem is the determination of the incubation period for the method.
We previously proposed the film-MPN method for the solid environmental sample (Song 2001). In order to construct the film-MPN method applying for aquatic environmental sample, we studied the rule of positive tube appearing, and determined the incubation time for the method.
Fig. 3 shows that the relationships between the numbers of positive-growth tubes and incubation time. 1 ml of 10-4-fold diluted river water and 10-6-fold diluted garden soil, river bank soil and activated sludge were inoculated into each of three groups of tubes. Every group included 75 tubes. In every tube there were 5 ml of growth medium and a piece of PHB/V film, and the tubes were incubated at 28 0C in the dark under
aerobic conditions.
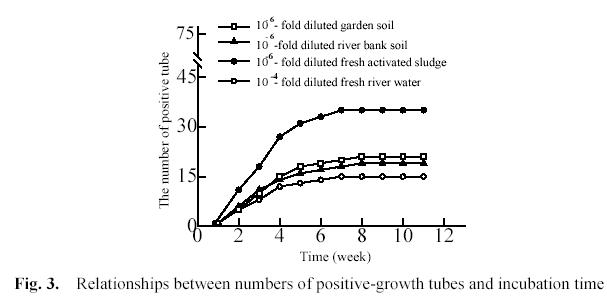
We used previously the First-Order Reaction (FOR) model to determine the incubation time for soil environmental sample. The expected final numbers were close to the numbers observed after incubating for 9 weeks in the soil 1 group and soil 2group (Song 2001). In fact, the numbers of positive tubes did not change for soil environmental sample after 8 weeks. Fig. 3 showed the numbers of positive tubes did not change for aquatic environmental sample after 7 weeks. Therefore, we determined incubation time was 8 weeks for aquatic environmental sample in film-MPN method.
Mode of growth and detection of positive-growth tubes
After a week, the solutions in some of the tubes inoculated with different environmental samples became opaque. Positive-growth was detected by observation of the film pieces in the tube. When each tube was lightly shaken by hand, small pieces of film appeared after 7 days of inoculation with river water and after 6 days with activated sludge. After tubes were further incubated for 2 or 3 weeks, pigment deposit, such as
yellow color, began to appear on some films near the solution surface in tubes inoculated with the soil samples. Also, a green color began to appear in tubes inoculated with the river sample. Some of the films near the surface of the solution in the positive-growth tubes appeared to be broken after incubating for 4 weeks in the gardensoil, paddy field soil, infertile garden soil and river bank soil groups, after 3.5 weeks in
the river water and seawater groups, and after incubating for 3 weeks in the activated sludge group. Obviously, the growth rates of PHB/V degraders from aquatic ecosystemswere faster than those from soil samples.
The positive-growth tube was the tube in which PHB/V-film was degraded by PHB/V degraders. Small pieces of PHB/V-film appeared and/or the films near the surface of the solution appeared to be broken in the positive-growth tube.
Numbers of PHB/V degraders in different environments
Through preliminary experiments, it was decided to use ten-fold dilution for estimating the numbers of PHB/V degraders in soil, activated sludge and river water.Numbers of PHB/V degraders in natural environments estimated by the film-MPN method are shown in Table 1. The numbers of PHB/V degraders differed greatly depending on differences in natural surroundings. In infertile garden soil, river bank soil,
garden soil, field paddy soil and farm soil, the numbers ranged from 1.6×104/g dry soil to 8.71×105/g dry soil while the number in river water and activated sludge was 2.2×103/ml and 5.14×105/ml, respectively. In a preliminary experiment, the number of PHB/V degraders in seawater was between 102 and 103. Therefore, a two-fold dilution was used to estimate the number of degraders in seawater. The number was estimated tobe 2.68×102/ml.
The numbers of PHB/V degraders in different kinds of environments estimated bythe film-MPN method were compared with those estimated by the clear-zone technique.The differences of numbers estimated by the two methods were ≦ ± 10.5 %.Interestingly, the numbers of PHB/V degraders in aquatic ecosystems estimated by the film-MPN method, such as river water, aerobic sewage sludge and seawater, were
higher than those estimated by the clear-zone technique. These results indicated that thefilm-MPN method was more suitable than the clear-zone technique for estimating the numbers of PHA degraders in aquatic ecosystems.
Changes of PHB/V weight before and after biodegradation
Fig. 4 shows the weight changes of injection molded PHB/V test pieces. The weight remaining after 30 days was 93.01 % in activated sludge, 97.51% in river water,97.98% in seawater, 99.13% in farm soil and 99.72% in infertile soil, respectively. After 480 days, the weight remaining was 10.87 % in activated sludge, 80.44% in river water,83.96% in seawater, 84.32% in farm soil and 98.88% in infertile soil, respectively.
Farm soil and infertile garden soil belong to similar ecosystem in different regions. The number of PHB/V degraders in farm soil was higher ( 8.71×105 /g, dry-soil) than that in infertile garden soil (1.6×104/g, dry-soil). Obviously, the results showed that biodegradability of PHB/V related to numbers of PHB/V degraders in similar environments in different regions.
After more than 60 days, it was found that smooth, sticky and thin layers were formed on the surfaces of test pieces in various kinds of aquatic ecosystems, such as river water, seawater and activated sludge. We name them as conditioned film and they were consisted of microorganisms and organic substances. The conditioned films as carrier of degraders could increase the biodegradability of PHB/V test pieces. They
could protect proliferation of degraders and prevent PHA-depolymerases secreted by degraders from flowing out to aquatic ecosystem. The test pieces were cleaner and evener in aquatic ecosystem than in soil ecosystem after biodegradation. This was attributed to the conditioned films, which were cleaned out of the test pieces after being washed with 75% alcohol and water.
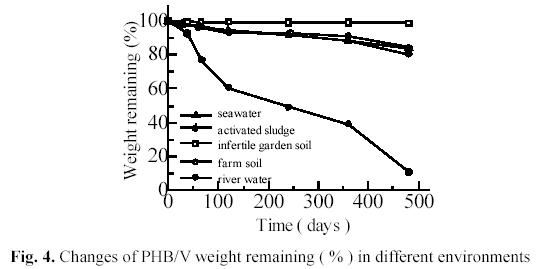
Fig. 4 also shows that the degradability of PHB/V in aquatic ecosystems is considerably higher than in soil ecosystem. One of the possible reasons is that degradation process of PHB/V is hydrolytic reaction of polyester under catalysis of PHA-depolymerase secreted by degraders on the polymer. Since PHA-depolymerase secreted by degraders were water-soluble, the enzymic degradation was more easily to
take place in aquatic ecosystem than in soil ecosystem.
Fig. 4 shows the degradability in activated sludge is highest in three kinds ofaquatic ecosystems. It is considered that the number of PHB/V degraders in activated sludge is the highest in the aquatic ecosystems. The degradation rate in seawater shows a little slower than that in river water. It is probably due to the fact that there were less degraders in seawater than in river water. These results agree with the numbers of
degraders estimated by the film-MPN method (5.14×105/mL activated sludge, 2.20×103/mL river water, 2.68×102/mL seawater).
Conclusions
The modified film-MPN method can be used to estimate the numbers of PHA degraders in different natural environments. Although the cultivation period used in the film-MPN method is longer than that used in the clear-zone technique, visual judgment of the results obtained by using the film-MPN method is simple and only a small amount of PHA sample (approximately 0.75 - 1.0g) is needed. Even if the PHA cannot
be made into milky suspension of powder, neither emulsifier nor surfactant is required for the film-MPN method. The film-MPN method is more suitable than the clear-zone technique for the samples obtained from aquatic ecosystems. Theoretically, provided cultivation period and the component of medium were determined, the film-MPN method is applicable to all environmental biodegradable materials that can be processed into films. On the other hand, the results showed that biodegradability of PHB/V related
to numbers of PHB/V degraders in similar environments in different regions. In different kinds of environments, such as soil, river water and activated sludge, the biodegradability of PHB/V not only related to the number of PHB/V degraders but also depended on whether there were conditions for the PHB/V degraders to grow and proliferate easily in the environment.
Acknowledgements
This work is supported by Natural Science Foundation of China (No. 20374032).
Reference
1. K. Sudesh, H. Abe, Y. Doi (2000) Prog. Polym. Sci. 25, 1503-1555.
2. Wendy Amass, Allan Amass & Brian Tighe (1998) Polymer International 47, 89-144.
3. A. Chowdhury (1963) Arch. Mikrobiol. 47, 167-200.
4. F. P. Delafield, M. Doudoroff, N. J Palleroni,. C. J. Lusty, and R. Contopoulos (1965) J.
Bacteriol. 90, 1455-1466.
5. J. Mergaert, A. Webb, C. Anderson, A. Wouters, and J. Swings (1993) Appl. Environ.
Microbiol. 59, 3233-3238.
6. A. Schirmer, D. Jendrossek, and H. G. Schlegel (1993) Appl. Environ. Microbiol. 59,
1220-1227.
7. H. Brandl, R. Bachofen, J. Mayer, and E. Wintermantel (1995) Can. J. Microbiol. 41(Suppl. 1)
143-153.
8. D. Jendrossek, A. Schirmer, and H. G. Schlegel (1996) Appl. Microbiol. Biotechnol. 46,
451-463.
9. C. R. Hankermeyer, and R. S. Tjeerdema (1999) Rev. Environ. Contam. Toxicol. 159, 1-24.
10. A. Steinbüchel (1996) In Rehm HJ et al. (Eds) Biotechnology, vol 6, VCH, Weinheim,
405-451.
11. D. Jendrossek, I. Knoke, R. B. Habibian, A. Steinbüchel, and H. G. Schegel (1993) J. Environ.
Polym. Degrad. 1, 53-63.
12. B. H. Briese, D. Jendrossek, and H. G. Schlegel (1994) FEMS Microbiol. Letter 117, 107-112.
13. J. Mergaert, A. Schirmer, L. Hauben, M. Mau, B. Hoste, K. Kersters, D. Jendrossek, and J.
Swings (1996) Int. J. Syst. Bacteriol. July, 769-773.
14. M. Matavulj and H. P. Molitoris (1992) FEMS Microbiol. Rev. 103, 323-332.
15. H. Nishida, and Y. Tokiwa, (1993) J. Environ. Polym. Degrad. 1, 227-233.
16. H. Nishida, S. suzuki and Y. Tokiwa (1998) J. Environ. Polym. Degrad. 6, 43-58.
17. D. M. Horowitz, and J. K. M. Sanders (1995) Can. J. Microbiol. 41 (Suppl. 1), 115-123.
18. B. A. Ramsay, I. Saracovan, , J. A. Ramsay, and R. H. Marchessault (1994) J. Environ.
Polym. Degrad. 2, 1-7.
19. R. H. Marchessault, F. G. Morin, S. Wong, I. Saracovan (1995) Can. J. Microbiol. 41
(Suppl.1), 138-142.
20. A. Schirmer, C. Matz, and D. Jendrossek, (1995) Can. J. Microbiol. 41 (Suppl 1), 170-179.
21. C. J. Song, U. Uchida, S. Ono, C. Shimasaki and M. Inoue (2001)
Biosci. Biotechnol. Biochem. 65, 1214-1217.
22. C. J. Song, S. F. Wang, S. Ono, B. H. Zhang, C. Shimasaki and M. Inoue (2002) Soil Sci. Plant
Nutr. 48, 159-164.
23. C. J. Song, S. F. Wang, S. Ono, B. H. Zhang, C. Shimasaki, and M. Inoue (2003) Polym. Adv.
Technol. 14, 184-188.
24. W. Mizuno, M. Kawaguchi, N. Sarukura, I. Omodaka, and S. Takeguchi (1996) KOBUNSHI
RONBUNSHU, 53, 513-521.
25. W. Mizuno, Y. Maeda, K. Kozima, T. Takamichi, K. Miyabe, and S. Takeguchi (2001)
KOBUNSHI RONBUNSHU, 58, 59-65.
26. The Committee of Standard Method of Soil for Analysis and Measurement (1986) in Japan
Society for Soil and Fertilizer Science (Eds), The Standard Method of Soil for Analysis and
Measurement, HAKUTOMOSHYA, Japan, 70-175.
27. K. P. Caballero, S. F. Karel, and R. A. Register (1995) Int. J. Biol. Macromol. 17, 86-92.
28. S. Ishikuri (1992) in Japan Society for Soil Microbiology (Eds), New Compilation Soil
Microbiology Experimental Method, YOKENDO, Tokyo, 45-54.
29. T. Hattori (1985) Rep. Inst. Agric. Res. Tohoku Univ., 34, 1-36.
30. S. Ishikuri, Y. Suwa, and T Hattori (1984) Soil Sci. Plant Nutr., 30, 249-253.
31. W. G. Cochran (1950) Biometrics, 6, 105-11
自然条件下降解菌数量与生物降解性的关系研究
摘要:为了评估自然环境对生物可降解材料降解的影响,改进了早前采用的薄膜-MPN 法,用以测定不同环境下降解PHB/V 的微生物(降解微生物)数量。研究了培养与阳性管之间的关系以确定该方法适当的培养期。采用薄膜-MPN 法在园土、水田土、农田土、河岸土、贫瘠园土、河水、活性淤泥以及海水中测定了需氧的PHB/V 降解微生物的数量。将结果与采用清亮区法的测定结果比较表明,薄膜-MPN 法适合于在环境测试中测定PHB/V 降解微生物的数量。另一方面,在多种环境中研究了注塑PHB/V 试样的生物降解性。对重量变化的研究表明PHB/V 的降解性与同一生态体系中不同区域PHB/V 降解微生物的数量有关。而在不同的环境中,PHB/V 的生物降解性不仅与PHB/V 降解微生物的数量有关,还取决于环境是否适宜于PHB/V 降解微生物的生长和繁殖。
论文来源:1st International Conference on Technology and Application of Biodegradable Polymers and Plastics,October,2004