废聚对苯二甲酸乙二醇酯(PET)可通过定向加氢转化为高价值化学品,展现出巨大的应用潜力。然而,实现精准的催化控制仍面临挑战。在本研究中,研究人员首次利用精心设计的 PtW/MCM-48 催化剂,通过精准调控催化剂的中等酸性位点,实现了废 PET 一锅法选择性加氢制备对甲基苯甲酸(p-TA),产率高达53.4%,同时联产36.4%的对二甲苯(PX)。机制研究表明,其优异的催化性能源于 Pt 纳米颗粒与 WOx 物种之间的协同作用:低价 WOx 物种增强了 Pt 的分散性,而 Pt 进一步稳定 WOx 为低聚合态多钨酸盐。PtW1.5/MCM-48 具备适度酸位点,能有效调控 p-TA 的解吸,抑制其过度加氢转化为 PX,并在真实 PET 废料转化中展现出良好的适用性。LCA 和 TEA 分析进一步凸显了该技术的应用前景。
新型化学回收技术被认为是一种实现废弃PET的高效转化和高值利用的理想方法。众所周知,对甲基苯甲酸(p-TA)是一种重要的化工原料,广泛用于感光材料、酰胺类杀菌剂的有机合成。目前,p-TA的工业生产仍依赖于以石油脑催化重整的对二甲苯(PX)为原料,通过浓硝酸氧化或环烷酸钴催化的传统石油途径,存在设备腐蚀严重、p-TA产率低、催化剂无法回收等棘手问题。相比之下,新型化学回收技术在传统的化学回收工艺的基础上,原位升级解聚后的单体,但PET分子结构中苯环与羧基的共轭效应导致其不对称加氢反应极为困难,实现不对称氢化产物的高产量仍然是一个重大挑战。克服这些内在障碍需要开发高度针对性和选择性的催化系统,其中催化剂的适度酸度设计呈现出非同寻常的复杂性和难度。
本文亮点
1、本研究采用一步法选择性加氢策略,实现废 PET 高选择性不对称加氢定向制备高值化学品 p-TA,适用于多种形式的 PET 废料。生命周期评价(LCA)和技术经济评价(TEA)结果表明,该方法不仅显著减少了传统石化工艺带来的环境污染,同时具备优越的经济可行性。
2、本研究采用精心设计的双功PtW/MCM-48催化剂,展现出卓越的催化性能,使 p-TA 和 PX 的收率分别达到 53.4% 和 36.4%。催化剂具有较高的稳定性,易分离、可循环利用。
3、多种表征手段和DFT计算结果共同表明,PtW/MCM-48催化剂的优越性能源于Pt NPs和WOx物种之间的协同相互作用,优化的 Pt/W 比例赋予催化剂适度的中等酸性位点,可以精准控制中间体的吸附和解吸,从而促进p-TA的形成,同时抑制过加氢生成PX。
4、本研究开发了一种高效的金属/酸双功能催化剂,深入解析了废 PET 选择性加氢过程中羰基环和苯环的部分氢化机制,对废塑料氢化回收新技术的开发具有重要参考价值。
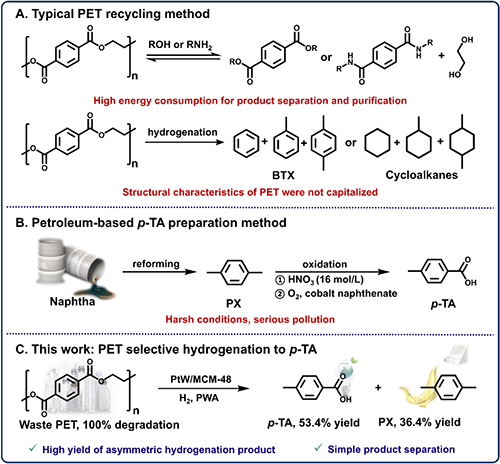
Figure 1. Schematic diagrams of conventional PET recycling methods, petroleum-based p-TA preparation, and the new method for p-TA production via selective hydrogenation of PET in this work.
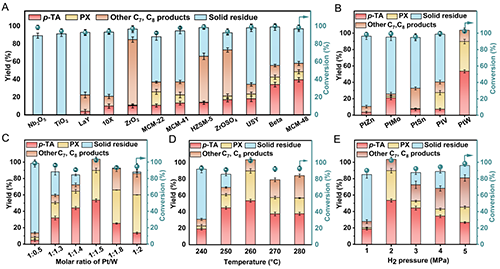
Figure 2. Optimization of reaction conditions for targeted hydrogenation of waste PET to p-TA and PX over Pt-based catalysts. (A) Effect of different supports; (B) effect of second metal sites of bimetallic Pt-M/MCM-48 (n(Pt): n(M) = 1:1.5); (C) effect of Pt/W molar ratio in PtW/MCM-48 catalyst; (D) effect of reaction temperature; (E) effect of H2 pressure. General reaction conditions: 0.05 g PET, 3 mL of H2O, 12 h, 700 rpm, 0.05 g catalyst with 5.0 wt% Pt loading, pH=2, 260 °C, 2 MPa H2.
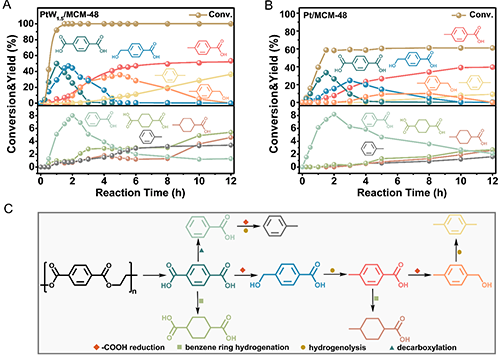
Figure 3. (A, B) Reaction product distributions on the targeted hydrogenation of waste PET over (A) PtW1.5/MCM-48 and (B) Pt/MCM-48 catalysts as a function of time. Reaction conditions: 0.05 g PET, 3 mL of H2O, 260 °C, 2 MPa H2, 12 h, 700 rpm, 0.05 g catalyst, pH=2 (with PWA). Specially, for aromatic liquid products, the yields were calculated by (moles of aromatic ring in product)/ (moles of aromatic ring in feedstock). For raw PET materials, the conversion was calculated by (moles of all aromatic liquid products)/ (moles of aromatic ring in feedstock). (C) Possible reaction pathway for the targeted hydrogenation of waste PET over PtW1.5/MCM-48 catalyst.
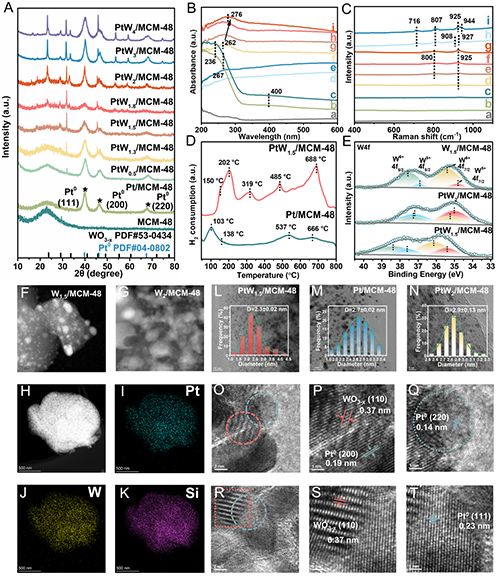
Figure 4. (A) XRD patterns of MCM-48, Pt/MCM-48 catalyst, and PtW/MCM-48 catalysts with different Pt/W molar ratios. (B) UV-vis spectra of (a) MCM-48, (b) W1.5/MCM-48, (c) W2/MCM-48, (d) Pt/MCM-48, (e) PtW0.5/MCM-48, (f) PtW1.3/MCM-48, (g) PtW1.5/MCM-48, (h) PtW1.8/MCM-48, (i) PtW2/MCM-48. (C) Raman spectra of (a) MCM-48, (b) Pt/MCM-48, (c) PtW0.5/MCM-48, (d) PtW1.3/MCM-48, (e) PtW1.5/MCM-48, (f) PtW1.8/MCM-48, (g) PtW2/MCM-48, (h) W1.5/MCM-48, (i) W2/MCM-48. (D) H2-TPR profiles of the Pt/MCM-48 and PtW1.5/MCM-48 catalysts. (E) XPS spectra of W4f in the W1.5/MCM-48, PtW2/MCM-48 and PtW1.5/MCM-48 catalysts. (F, G) HAADF-STEM images of (F) W1.5/MCM-48 and (G) W2/MCM-48 samples. (H-K) EDX spectroscopy mapping profiles of PtW1.5/MCM-48 catalyst. (L-N) HR-TEM images and particle size distributions of (L) PtW1.5/MCM-48, (M) Pt/MCM-48, (N) PtW2/MCM-48 catalysts. (O-T) HR-TEM images of the PtW1.5/MCM-48 catalyst.
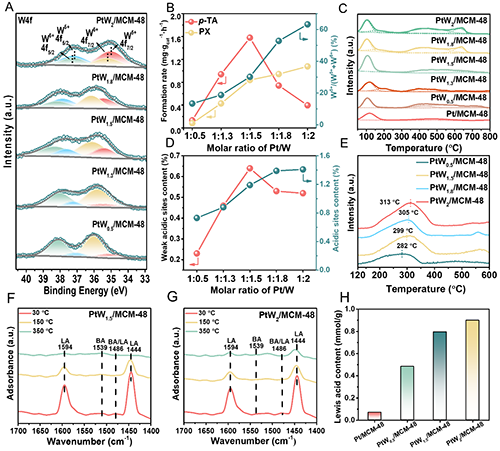
Figure 5. (A) XPS spectras of W4f in the PtW/MCM-48 catalysts with different PtW molar ratios. (B) Correlation between the molar ratio of Pt/W of PtW/MCM-48 catalysts and the formation rate of p-TA and PX, along with the W5+/(W5++W6+) ratio on the catalysts surface. (C) NH3-TPD profiles of the PtW/MCM-48 catalysts. (D) Relationship between Pt/W molar ratio in PtW/MCM-48 catalysts and the content of weak and total acidic sites. (E) Acetic acid TPD-MS results for PtW/MCM-48 catalysts with different PtW molar ratios. (F, G) The IR spectra of adsorbed pyridine on (F) PtW1.5/MCM-48 and (G) PtW2/MCM-48 catalysts. (H) Br?nsted and Lewis acid sites contents and ratios of Pt/MCM-48 and PtW/MCM-48 catalysts.
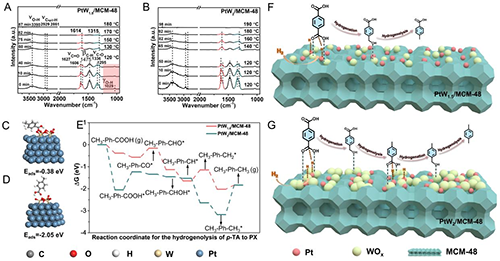
Figure 6. (A, B) DRIFTS images of acetic acid adsorption on (A) PtW1.5/MCM-48 and (B) PtW2/MCM-48 catalysts. (C, D) The intermediate CH3-Ph-COOH* on the (C) W3O7/Pt(111) and (D) W4O7/Pt(111) model. (E) Reaction energies of the hydrogenolysis of p-TA on W3O7/Pt(111) and W4O7/Pt(111) surfaces. (F, G) Schematic diagram of selective hydrogenation of waste PET to p-TA and PX over (C) PtW1.5/MCM-48 and (D) PtW2/MCM-48 catalysts.
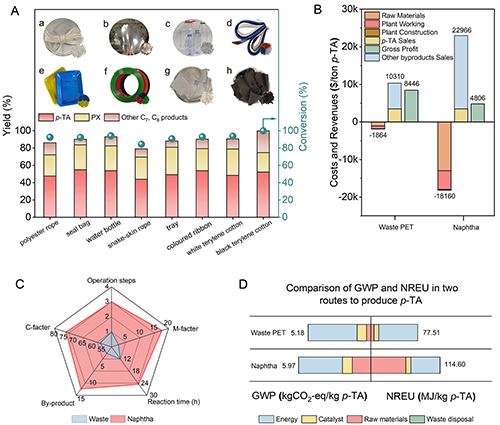
Figure 7. (A) Targeted hydrogenation of real waste PET plastics (a) polyester rope, (b) seal bag, (c) water bottle, (d) snake-skin rope, (e) tray, (f) coloured ribbon, (g)white terylene cotton, (h)black terylene cotton to p-TA and PX over PtW1.5/MCM-48 catalyst. General reaction conditions: 0.05 g real PET plastics, 3 mL of H2O, 260 °C, 2 MPa H2, 700 rpm, 12 h, 0.05 g PtW1.5/MCM-48, pH=2 (with PWA). (B) Comparison of the revenues and costs of waste PET and naphtha to p-TA (100,000 tons p-TA production per year). (C) Comparison of the viabilities of the production of p-TA from waste PET and naphtha. “M-facter” referred as Material consumption (ton/ton p-TA) and “C-facter” referred as Catalyst cost ($/ton p-TA). (D) Comparison of non-renewable energy use (NREU) and global warming potential (GWP) in production of 1 kg p-TA from waste PET and naphtha.
原文信息:
第一作者:朱越、毛周颖
通讯作者:梅清清
通讯单位:浙江大学环境与资源学院
论文DOI:10.1021/jacs.5c01209
https://pubs.acs.org/doi/10.1021/jacs.5c01209
通讯作者简介

梅清清,浙江大学环境与资源学院“百人计划”研究员,博士生导师,英国皇家学会牛顿国际学者。长期致力于生物质/废塑料等有机固体废弃物的高值化利用,围绕关键化学键的精准活化与调控,开发原子经济性转化路线与绿色催化体系,以实现复杂多介质有机固废体系的高选择性资源回收。在Sci. Adv., J. Am. Chem. Soc., Angew. Chem. Int. Ed. 等权威期刊上发表SCI论文50余篇,授权国家发明专利13件,主持国家自然科学基金、浙江省重点研发计划等项目。担任Science Bulletin/《科学通报》 特邀编委,The Innovation期刊青年编委,Carbon & Hydrogen青年编委。
主要研究方向:废塑料/生物质等固废资源化;有机固废基环境功能材料;多源固废协同治理技术与装备。
E-mail: meiqq@zju.edu.cn
教师主页:https://person.zju.edu.cn/qingqingmei
- 美国华盛顿州立大学雷寒武副教授等开发一种将日常塑料废物高效转化为航空煤油的新技术 2019-06-05
