Baoqing SHENTU, Quan LIU, Qing ZHU, Zhixue WENG
(Department of Chemical Engineering, Zhejiang University, Hangzhou 310027, China)
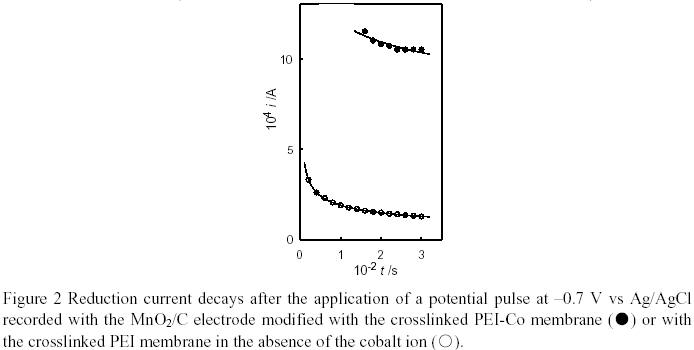
Poly(ethyleneimine)-cobalt (PEI-Co) complex displayed rapid and reversible oxygen-binding properties, and can be used as an efficient oxygen carrier1-4. Howerever, PEI is water-soluble, which influences the application of the PEI-Co complex in the modification of oxygen electrode. Since polyepicholohydrin (PECH) possesses moderately active chloromethyl groups, PECH was used to react with imino groups in PEI to prepare the crosslinked membrane. An insoluble and swellable membrane of a cobalt complex with a crosslinked PEI ligand can expect to serve as an oxygenenriching material immobilized at the electrode surface to concentrate oxygen from aqueous electrolyte solutions5-7. Here, we report the preparation of the PEI-Co complexed membrane and the effect of the membrane on the reduction current of the modified oxygen electrode.
A cobalt complex with a cross-linked PEI ligand was prepared according to the method described in the literature5 with slight modifications as follows. A solution of PECH and PEI in DMF was heated to 60 ℃ slowly enough to prevent gelation, and then the hot solution was transferred onto the surface of a glassy plate. The solvent was allowed to evaporate at 100 ℃ for 2 h. Washing the product with methanol followed by drying under vacuum afforded the transparent membrane of the crosslinked PEI ligand. The membrane was dipped into the cobalt(II) chloride solution to complex the cobalt ions to the imino groups. The amount of cobalt ions in the polymeric complex was estimated by spectral analysis and a Nikko Keisoku DPGS-1 dual potentiogalvanostat and a Nikko Keisoku NFG-3 universal programmer were employed to obtain the current decay curves in potential step experiments.
It was found that the properties of the crosslinked PEI membrane related to the ratios of PEI to PECH. With an increase of the PEI content, the membrane displayed an outstanding swelling property in methanol or water, and poor mechanical properties.
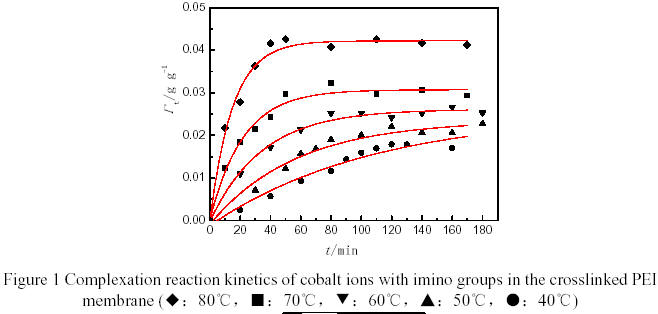
Figure 1 showed the relationship between the amount of cobalt ions complexed with imino groups in the crosslinked PEI membrane (Γt) and time (t). It was found that Γt obeyed the first order kinetics curve, and the higher of the temperature, the rapider of the complex reaction. The activation energy of the complex reaction was estimated to be 62.14 kJ/mol according to the change of the reaction rate with the temperature.