Alejandro J.Mtlller, Julio Albuerne, Marcos Sabino and Leui Marquez
Grupo de Polimeros USB, Departamento de Ciencia de los Materiales, Universidad Sim6n Bolivar,
Caracas 1080-A, Venezuela. E-mail: amuller@usb.ve.
Poly(p-dioxanone) or PPDX is a synthetic poly(ester-ether) that exhibits appropriate molecular structure and properties for biomedical applications such as suture material, pins for fracture fixation, surgical clips and fasteners1-2. It is well established that PPDX can be degraded by hydrolysis generating low molecular weight hydroxy-acid species that can be metabolized or bioabsorbed by the human body1-2 The present work reviews our previous investigations into the morphology, nucleation, crystallization kinetics and properties of neat and hydrolytically degraded PPDX and PPDX-b-PCL diblock copolymers3-10. We have studied the hydrolytic degradation of high molecular weight PPDX suture monofilaments. It was demonstrated that the hydrolytic degradation of PPDX occurs in a two stage process where the amorphous regions of the sample are attacked faster than the crystalline regions of the sample. The changes experienced by the samples as degradation proceeded were successfully monitored by viscometry, Differential Scanning Calorimetry (DSC), weight loss, pH changes, Scanning Electrom Microscopy (SEM) and Atomic Force Microscopy (AFM). A schematic morphological model has been proposed that is consistent with the classical "Swiss cheese" model and with the data collected.
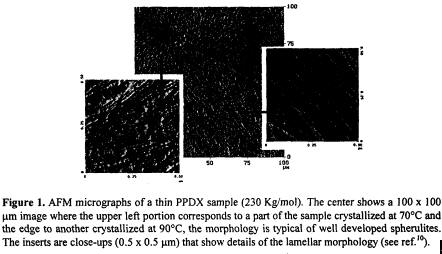
The behavior of the lamellar thickness as a function of isothermal crystallization temperature was examined by AFM (Fig. 1) together with melting point data obtained by DSC. Through the use of the Thompson-Gibbs equation we were able to obtain, a value of the fold surface free energy for PPDX, i.e., 166 erg/cm2. This value is in the same range as those obtained previously for similar linear polyesters.
The PPDX-b-PCL diblock copolymers employed are most probably in a weak segregation regime and we have recently shown that crystallization drives structure formation9, whereby lamellar structures of PPDX and PCL are formed. Depending on composition well developed banded spherulites can be observed7-8. The two blocks crystallize in a single coincident exotherm when the material is cooled from the melt in the DSC. We have demonstrated7-9 that such a coincident crystallization is due to the retardation of the PPDX crystallization because it is covalently bonded to PCL. The self-nucleation technique is able to separate into two exotherms the crystallization of each block by self-nucleating just the PPDX component8-9. Furthermore, we have gathered evidences indicating that the PPDX block can nucleate the PCL block within the copotymers regardless of the composition. These evidences are: 1) If a PPDX-b-PCL copolymer is quenched to a temperature at which both blocks can crystallize and the isothermal crystallization is followed, the overall crystallization rate is slower than when PPDX is crystallized first and the material is later crystallized under the same conditions7. 2) When PPDX is isothermally crystallized at high temperatures and then the sample is quenched, the crystallization of the PCL component occurs even faster than an equivalent PCL homopolymer of the same Mw, for compositions where PCL is the excess component7-9. 3) For certain compositions, after self- nucleation of the PPDX block, a nucleating effect is detected in the PCL block as evidenced by a shift of the crystallization temperature to higher values8-9.
Even for compositions where only 23% PCL is present in the copolymer, both the spherulitic growth rate and the overall crystallization rate experienced a strong depression as compared to a PPDX homopolymer of equivalent Mw in a temperature range where PCL is molten (i.e., at crystallization temperatures above 55℃). The Lauritzen and Hoffman theory was successfully applied to describe the energy barriers associated with the nucleation and the growth of PPDX homopolymer and the PPDX block within PPDX-b-PCL.9 The nucleation effect of the PCL by the PPDX is responsible for the lack of homogeneous nucleation or fractionated crystallization of the PCL block even when it constitutes a minor phase within the copolymer (25% or less). Nevertheless, we were able to show that increasing amounts of PPDX within the diblock copolymer still produces topological restrictions that retard the crystallization kinetics of the PCL component and decrease the Avrami index obtained during isothermal crystallization, even if the nucleation is always heterogeneous.8-9
Finally, preliminary results on hydrolytic degradation showed that the presence of relatively small amounts of PCL within PPDX-b-PCL copolymers substantially retards hydrolytic degradation of the material in comparison to homo-PPDX. This increased resistance to hydrolysis is a complex function of composition and its knowledge may allow the prediction of the lifetime of the material for biomedical applications.9
REFERENCES
(1) Yang, K.K.; Wang,X.L.; Wang, Y.Z.J.Macromol.Sci., Part C,Polym.Rev. 2002, C42, 373 (and references therein).
(2) Bezwada, R.S.; Jamiolkowski, D.D.; Cooper,K.In Handbook of Biodegradable Polymers; Domb, A.J.; Kost,J.; Wiseman,D.M.; Eds.; Harwood: Singapore, 1997; Chapter 2, pp 29-61.
(3) Sabino, M.A.; Ronca,G.; Müller,A.J.J.Mat. Sci. 2000, 35, 5071.
(4) Sabino, M. A.; Feijoo,J.L.;Müller,A.LMacromol.Chem.Phys.2000,201,2687.
(5) Sabino, M. A.; Feijoo,J.L.; Müller,A.J.Polym. Degrad Stab.2001,73,541.
(6) Sabino M. A.; Sabater L.; Ronca G.; Müller A. J. Polymer Bulletin, 2002, 48, 291.
(7) Albueme J; Márquez L.; Müller A. J.; Raquez J. M.; Degée Ph.; Dubois Ph.; Castelletto V.; Hamley I. Macromolecules, 2003, 36, 1633.
(8) Müller A. J.; Albuerne J.; Esteves L. M.; Márquez L.; Raquez J. M.; Degée Ph.; Dubois Ph.; Collins S. and Hamley I. W., Macromolecular Symp., 2004, in press.
(9) Müller A. J.; Albueme J.; Marquez L.; Raquez J.-M.; Degée Ph.; Dubois Ph.; Hobbs J. and Hamley I.W., submitted to Faraday Discussion, 2004.
(10) M. A. Sabino, J. Albueme, A. J. Müller, J. Brisson and R. E. Prud'homme, Biomacromolecules, 2004, 5,358.
论文来源:PP’2004 Dail international symposium on Polymer Physics Preprints,june 1-5,2004,Dail,china