S. Penczek*, J. Pretula, and K. Kaluzynski
Centre of Molecular and Macromolecular Studies, Polish Academy of Sciences, 90-363 Lodz, Sienkiewicza 112, Poland
Keywords: poly(alkylene phosphates), bio mimicking polymers, liquid membranes, crystal growth modifiers, polyaniline
Poly(alkylene phosphate) backbones are at the basis of two important classes of biomacromolecules, nucleic and teichoic acids. Both are known to strongly bind metal cations; teichoic acids interact specifically with Ca2+ and Mg2+ cations transporting these cations in the biological milieu. The work of our laboratory directed towards synthesis of the backbones interaction with cations and some applications in non-biological systems related to the ability to interact with cations is presented. The following major synthetic methods have been elaborated and will be discussed:
- ring opening polymerization, [1] e.g.:
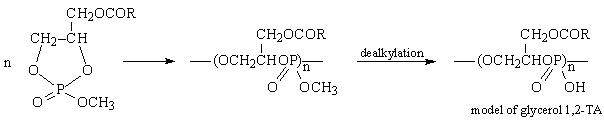
- polytransesterification [2]
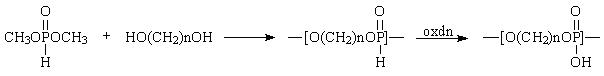
Poly(alkylene phosphates) were applied as cations transporting agents in liquid membranes [3] and in the form of block copolymers as crystals growth modifiers by interacting with cations rich surface. Particularly effective are di- and triblock copolymers allowing preparation CaCO3 particles of shapes, sizes, and sizes distribution depending on the copolymer composition and molar mass [4]. Similar polymers, prepared according to the methods elaborated in our laboratory, were used in the synthesis of injectable material for controlled drug and gene delivery [5].
Moreover, poly(alkylene phosphates) function as strong acids: cationation of polyaniline (doping) leading to the intermolecular complex, in which poly(pentamethylene phosphate) specifically recognize the distance between the nitrogen atoms in polyaniline [6].
[1] Penczek, S., et al., Models of Biopolymers Related to Nucleic and Teichoic Acid, S. Penczek Ed., CRC Press, Boca Raton, Florida 1990, p. 291-378.
[2] Penczek, S., et al., 1999. J. Polymer Sci. Part A: Polym. Chem. 37, 1365-1381.
[3] Wodzki, R., et al., J. Appl. Polymer Sci. 93, 1436-1445.
[4] Cölfen H., et al., 2002. Macromolecular Chem. Phys., 203, 627-635.
[5] Leong, K.W., et al., 2004. Macromolecules 37, 670-672.
[6] Pron, A., et al., 1994. J. Chem. Soc. Chem. Commun. 641-642.