Taming the wildness of “Trojan-Horse” peptides by charge-guided masking and protease-triggered de-masking for the controlled delivery of anti-tumor agents
作者:Shi NQ, Qi XR
关键字:“Trojan-Horse” peptides, taming the wildness, charge-guided masking, protease-triggered demasking, controlled delivery
论文来源:期刊
具体来源:ACS Appl Mater Interfaces. 2017. Mar 29;9(12):10519-10529. (SCI, IF=7.145)
发表时间:2017年
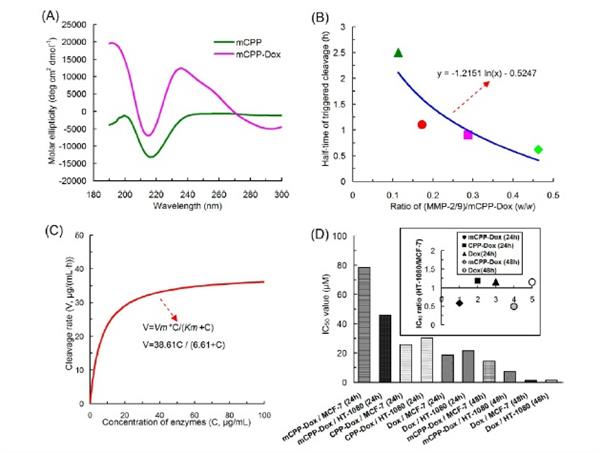
Cell-penetrating peptide (CPP), also called “Trojan Horse” peptide, has become a successful approach to deliver various payloads into cells for achieving the intracellular access. However, the "Trojan Horse" peptide is too wild, not just to "Troy", but rather widely distributed in the body. Thus, there is an urgent need to tame the wildness of “Trojan Horse” peptide for targeted delivery of antineoplastic agents to the tumor site. To achieve this goal, we exploit a masked CPP-doxorubicin conjugate platform for targeted delivery of chemotherapeutic drugs using charge-guided masking and protease-triggered de-masking strategies. In this platform, the cell-penetrating function of the positively CPP (D-form
nonaarginine) is abrogated by a negatively shielding peptide (masked CPP), and between them is a cleavable substrate peptide by the protease (MMP-2/9). Protease-triggered
de-masking would occur when the masked CPP reached the MMP-2/9-riched tumor. The
CPP-doxorubicin conjugate (CPP-Dox) and the masked CPP-Dox conjugate (mCPP-Dox)
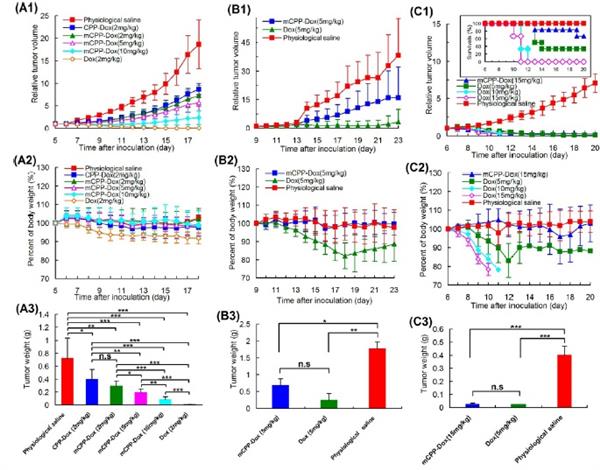
were used as models for the evaluation of masking and de-masking processes. It was found that exogenous MMP-2/9 could effectively trigger the reversion of CPP-cargo in this conjugate and this trigger adhered to Michaelis-Menten kinetics profile. This conjugate was sensitive to the trigger of endogenous MMP-2/9 and could induce enhanced cytotoxicity towards MMP-2/9-rich tumor cells. In vivo antitumor efficacy revealed that this masked conjugate had considerable antitumor activity and could inhibit the tumor growth at a higher level relative to CPP-cargo. Low toxicity in vivo showed the noticeably decreased
wildness of this conjugate towards normal tissues and more controllable entry of anti-tumor agents into “Troy”. Based on analyses in vitro and in vivo, this mCPP-cargo conjugate
delivery system held an improved selectivity towards MMP-2/9-rich tumors and would
be a promising strategy for tumor-targeted treatment.