Long-term electrocatalytic N2 fixation by MOF-derived Y-stabilized ZrO2: An insight into deactivation mechanism
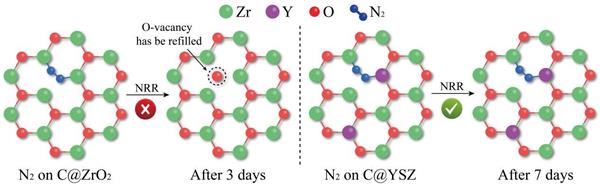
Industrially, NH3 synthesis largely dependent on the Haber-Bosch method which consumes a lot of energy and emits huge CO2. Recently, Electrochemical N2 reduction reaction (NRR) has been recognized as a promising method to achieve clean and sustainable NH3 production, thus the high-efficient and durable catalysts are urgently desired. In this paper, we report a MOF-derived carbon/Y-stabilized ZrO2 nanocomposite (C@YSZ) works as an efficient electrocatalyst for NRR in 0.1 M Na2SO4. It achieves a large NH3 production of 24.6 μ g h-1 mg-1 cat. and a high Faradaic efficiency of 8.2% at -0.5 V vs. reversible hydrogen electrode. Experimental results demonstrate the surface oxygen vacancies are the main catalytic sites for NRR.
Introducing of Y3+ into ZrO2 lattice has significant effect to increase and stabilize the O-vacancies. Meanwhile, this catalyst displays remarkable stability and durability for NRR performance, showing a negligible change after 7 days reaction, better than most reported NRR electrocatalysts. Moreover, in-situ electrochemical quartz-crystal microbalance (EQCM) was firstly applied in NRR field and successfully combined with density functional theory (DFT) calculations to reveal the deactivation mechanism.
https://doi.org/10.1039/D0TA01154A