- 武汉理工大学木士春团队Small Methods:Lewis酸熔盐刻蚀偶联CoIr纳米颗粒与MXene实现广pH范围的高效析氢
- 来源:木士春教授研究团队 个人网站 2024-12-10

原创 木士春教授课题组 eChemStore 2024年11月03日 09:30 上海
第一作者:周清渠,赵宏宇
通讯作者:木士春
通讯单位:武汉理工大学
Doi: 10.1002/smtd.202401449
1. 全文速览
本文设计了一种通过Lewis酸熔盐刻蚀制备MXene的策略,CoIr合金与MXene载体之间通过Ti-O-M键合方式实现金属-载体强相互作用,增强了催化材料的稳定性,并通过电荷调控促进电荷由金属向载体转移,降低了Ir过强的氢吸附能,从而共同实现了宽pH范围下的高效析氢:在碱性和酸性条件下,分别仅需34和50mV即可驱动10 mA cm-2的电流密度。
2. 背景介绍
MXene作为一类新兴的二维材料,具有高的比表面积和高导电性,以及良好的表面亲水性,在电化学领域具有广泛的应用。然而,MXene担载金属需要经历一个复杂的过程,其中刻蚀是关键的一步。常用的刻蚀手段主要是通过含氟溶液进行湿法刻蚀(HF,NH4F/LiF+HCl),而氟元素对于环境的危害性较大。相比之下,如果采用Lewis酸熔盐刻蚀策略则可以有效避免氟离子的参与,同时直接将金属离子转化为纳米颗粒作为活性位点。此外,金属-载体强相互作用不仅可以实现对金属粒子的锚定作用,而且还可调控界面电荷转移,优化中间体的吸附能,从而优化金属位点的吸附行为。
3. 图文解析
合成方法

Figure 1. (a) Schematic illustration of the synthetic process of CoM/MXene catalysts. (b) SEM image of CoIr/MXene. (c-e) AC-TEM image. (f) SAED image and (g) Element mapping image of CoIr/MXene.采用熔盐辅助法合成CoIr/MXene催化材料。其中,选用Ti3AlC2 MAX作为MXene前驱物;LiCl-KCl混合盐作为熔盐具有较低的熔点,可以在反应中提供液相环境;CoCl2·6H2O作为刻蚀剂置换Al,生成的AlCl3沸点为181℃,反应后挥发;同时,以IrCl3作为活性位点来源。扫描透射电镜图像(Figure 1b)显示出明显的层状结构,证明刻蚀成功,球差透射电镜图像(Figure 1d和e)以及EDS分析(Figure 1g)可以看出CoIr纳米颗粒被成功担载于MXene表面。
结构分析
 Figure 2. (a) Normalized Ir L3-edge XANES curves of CoIr/MXene, Ir foil and IrO2 baselines and (b) Co K-edge XANES curves of CoIr/MXene, Co foil, Co3O4 and Co2O3. (c)FT-EXAFS spectra for R-space of Ir L3-edge CoIr/MXene, Ir foil and IrO2. (d) Co K-edge CoIr/MXene, Co foil, Co3O4 and Co2O3, (e) FT-EXAFS spectra for E-space of Ir L3-edge CoIr/MXene, Ir foil and IrO2. f) Co K-edge CoIr/MXene, Co foil, Co3O4 and Co2O3. (g-i) Wavelet transform for EXAFS signals of Ir foil, IrO2 and CoIr/MXene.
Figure 2. (a) Normalized Ir L3-edge XANES curves of CoIr/MXene, Ir foil and IrO2 baselines and (b) Co K-edge XANES curves of CoIr/MXene, Co foil, Co3O4 and Co2O3. (c)FT-EXAFS spectra for R-space of Ir L3-edge CoIr/MXene, Ir foil and IrO2. (d) Co K-edge CoIr/MXene, Co foil, Co3O4 and Co2O3, (e) FT-EXAFS spectra for E-space of Ir L3-edge CoIr/MXene, Ir foil and IrO2. f) Co K-edge CoIr/MXene, Co foil, Co3O4 and Co2O3. (g-i) Wavelet transform for EXAFS signals of Ir foil, IrO2 and CoIr/MXene.
同步辐射测试结果展现出Ir-Co/C以及Ir-O配位,说明CoIr/MXene中的CoIr以合金形式存在(Figure 2c),Ir与MXene表面之间通过Ti-O-M键合;同时,电荷发生转移,使Ir处于离子态。
性能测试
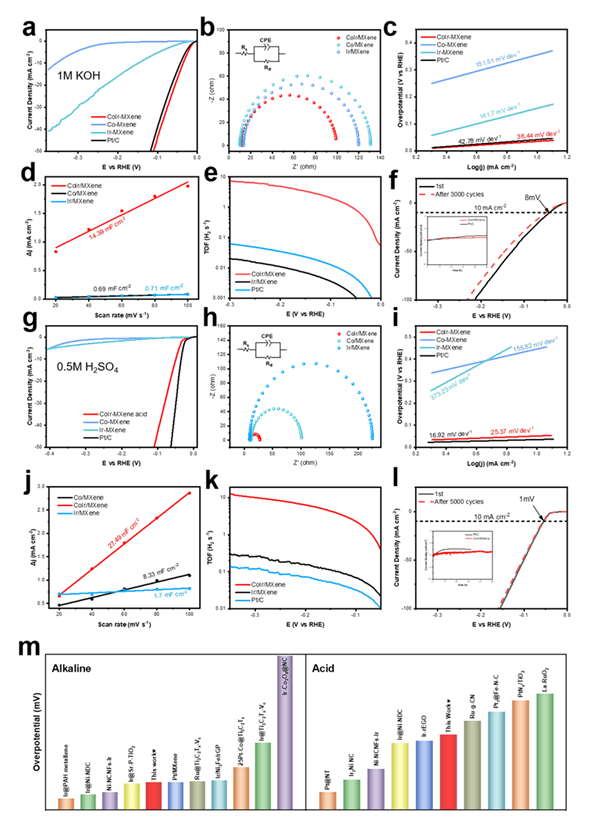
Figure 3. (a) LSV curves of CoIr/MXene, Co/MXene, Ir/MXene and Pt/C in 1M KOH. (b) Fitted EIS curves of catalysts in alkaline media. (c) Tafel plots derived from the LSV curves. (d) Liner fits of capacitive currents versus CV scan rates. (e) TOF curves of catalysts. (f) Demonstration of stability of CoIr/MXene after one and 3000 cycles and I-t test in illustration. (g) LSV curves of CoIr/MXene, Co/MXene, Ir/MXene and Pt/C in 0.5 M H2SO4. (h) Fitted EIS curves of catalysts in aidic media. (i) Tafel plots derived from the LSV curves. (j) Liner fits of capacitive currents versus CV scan rates. (k) TOF curves of catalysts. (l) Demonstration of stability of CoIr/MXene after one and 5000 cycles and I-t test in illustration. (m) Properties comparison of CoIr/MXene with reported noble metal catalysts.
测试结果表明,CoIr/MXene在酸性和碱性下均表现出优异的析氢反应(HER)性能,具有极低的过电位。在碱性和酸性电流密度为10 mA cm-2时过电位分别为34 和50 mV(Figure 3a和3g),在目前报道的催化剂中处于较优水平。Tafel斜率值表明HER的决速步骤由Heyrovsky步骤转变为Tafel步骤(Figure 3c和3i),改善了反应动力学。通过CV循环和50 h计时电流法测试表明催化剂具有优异的稳定性能(Figure 3f和3l及插图)。机理分析

Figure 5. In-situ Raman spectra of interfacial water on (a) CoIr/MXene and (b) Ir/C electrode in a 1 m KOH solution (E VS RHE). In situ Raman spectra of A1g peak on (c) CoIr/MXene and (d) Ir/C. The contact amgles of (e) CoIr/MXene and (f) CoIr/C. (g) The underwater bubble contact angle of CoIr/MXene. (h) Optimized structures of CoIr/MXene, (i) Charge density difference of CoIr/MXene, (j) Binding energy of CoIr/MXene and CoIr/C, (k) PDOS of Ir, C and O in CoIr/MXene, CoIr/C and Ir/C, (l)COHP of H adsorption on active sites for CoIr/MXene and CoIr/C, (m)Work function of CoIr/MXene and CoIr/C.
接触角的测试表明催化剂表面具有良好的亲水性以及疏氧性能,可以增强界面水吸附并减少氢气泡在表面的聚集。原位拉曼(Figure 5d-g)同样证实了催化剂具有较强的水吸附作用,在反应电压范围内K+水化水(KW)的含量明显上升;同时,A1g的峰强度也产生明显变化,表明表面含氧官能团(-O,-OH)也促进了反应进程。理论计算表明,电荷富集于MXene表面,提高了Ir的态密度,从而降低了Ir位点对于H的吸附,与同步辐射的表征结果相一致。4. 总结与展望这项工作采用熔盐刻蚀策略制备了MXene担载CoIr纳米合金催化剂。CoIr纳米颗粒通过Ti-O-M键方式锚定于MXene表面,使二者之间具有强相互作用。电荷由合金颗粒向载体发生转移,优化了中间体的吸附能,降低了Ir过强的氢吸附能和Volmer步骤的反应势垒,从而加速了反应动力学。此外,MXene良好的表面含氧官能团提供了优异的亲水性,促进了表面水吸附以及*OH的吸附。这些优势使得CoIr/MXene催化剂具有低的析氢过电位(碱性34 mV@ 10 mA cm-2,酸性50 mV@ 10 mA cm-2)以及良好的稳定性。本项研究成果为探索高效的MXene基催化剂提供了新思路。
5. 课题组介绍
武汉理工大学先进能源材料研究团队依托材料复合新技术国家重点实验室,长期从事质子交换膜燃料电池关键材料与核心器件、电化学产氢催化材料、锂离子电池电极材料和碳纳米材料等研究工作。欢迎有志于科技报国的研究生及博士后加入团队!
木士春研究团队主页http://shichunmu.polymer.cn/
- [来源:中国聚合物网]
- 了解更多请进入: 木士春教授研究团队 个人网站