- Boosting photo-self-Fenton-like reaction via ferric-ellagate complex for environmental remediation
- 来源:朱华跃教授个人网站 2024-02-23
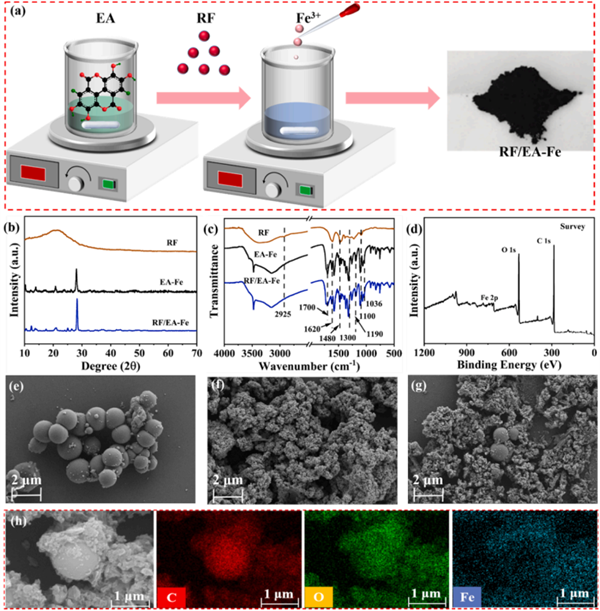
Efficient generation and rapid activation of H2O2 are essential for a self-sufficient photo- Fenton-like system. Herein, ferric-ellagate complex (EA-Fe), the atomically distributed Fe active
catalyst, was first employed to activate the H2O2 photo-generated by resorcinol–formaldehyde (RF) resins. During the photo-Fenton-like reaction, the hydroxyl radicals (?OH), superoxide radicals (?O2 – ), photoinduced holes (h+) and superoxide radical ( 1 O2) work together for organics degradation and bacteria inactivation. The degradation intermediates were identified by HPLC-EIS-MS and the possible degradation pathways of TC were proposed. Meanwhile, the QSAR prediction revealed that the catalytic processes could significantly eliminate ecotoxicity. Moreover, the RF/EA-Fe could effectively inactivate both E. coli and S. aureus. Theoretical calculations indicated that the H2O2 molecule was adsorbed on the Fe atom through a single Fe-O coordination, followed by conversion to *OH (Fe-OH) via directly breaking the peroxy bond. This activation pathway effectively maximizes the utilization of H2O2. This study can provide a new platform for rationally designing a self-cycled photo-Fenton-like system with outstanding degradation and antibacterial properties. https://www.sciencedirect.com/science/article/pii/S1383586624001898?dgcid=coauthor
- [来源:中国聚合物网]
- 了解更多请进入: 朱华跃教授个人网站